
The elements which belong to Group VA, VIA, and VIIA are non- metals. In conclusion, potassium is highly reactive than the sodium. As a result, potassium loses its valence electron more easily than sodium. Since the atomic size of sodium is smaller than the atomic size of potassium, sodium has more force of attraction between the nucleus and valence shell than that of potassium. As we move from top to bottom, the reactivity of metallic elements of Group IA increases.

They have one valence electron in their valence shell. Why is potassium highly reactive than the sodium?Īs sodium and potassium both are alkali metals that belong to Group IA of the periodic table. ↓ Reactivity increases from top to bottom Thus, the tendency of losing the valence electron/s increases and their chemical reactivity increases on moving from top to bottom in a group of metals. This is the reason why the bigger atom/s can lose the valence electron/s more easily than the smaller atom/s. While moving from top to bottom in a group of metals in the periodic table, the atomic size increases with the increment in the number of shells and the force of attraction between the nucleus and valence shell decrease. The reactivity of an element belonging to metals increase on moving from top to bottom in a group of the periodic table.

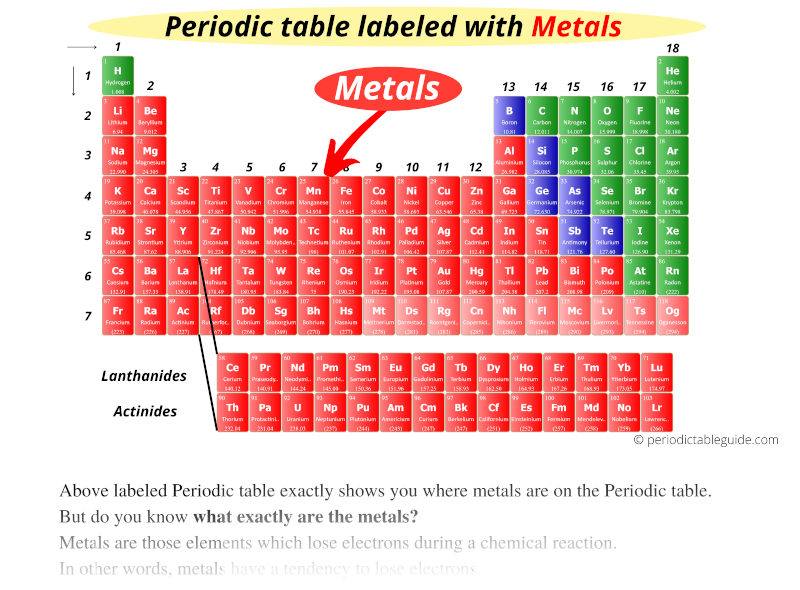
The elements which belong to Group IA, IIA and IIA are metals.


 0 kommentar(er)
0 kommentar(er)
